Baxter Reports 2017 Fourth-Quarter and Full-Year Results
Fourth-Quarter Revenue of $2.8 Billion Increased 5 Percent on a Reported Basis and 2 Percent on an Operational Basis
Fourth-Quarter GAAP Earnings Per Share (EPS) of ($0.11); Adjusted EPS of $0.64 Increased 12 Percent
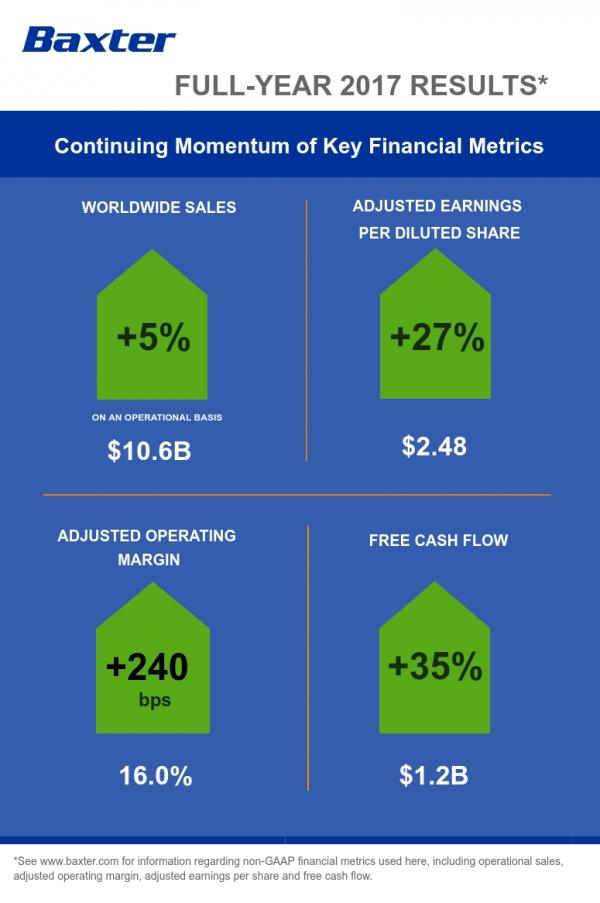
Full-Year Revenue of $10.6 Billion Increased 4 Percent on a Reported Basis and 5 Percent on an Operational Basis
Full-Year GAAP Earnings Per Share Were $1.30; Adjusted EPS of $2.48 Increased 27 Percent
Company Generated Full-Year Operating Cash Flow of $1.85 Billion and Free Cash Flow of $1.22 Billion
Deerfield, Ill. -
Baxter International Inc. (NYSE:BAX) today reported results for the fourth quarter and full-year ended December 31, 2017, and provided its financial guidance for 2018.
“Baxter’s solid performance in 2017 reflects the ongoing impact of strategic, disciplined execution,” said José (Joe) E. Almeida, chairman and chief executive officer. “We are combining an unwavering focus on operational excellence with an increased emphasis on innovation and portfolio expansion to deliver positive results for patients and investors. Looking ahead, our continued transformation will help support our aspiration of delivering industry-leading performance for our investors and other stakeholders in 2018 and beyond.”
Fourth-Quarter Financial Results
In the fourth quarter, worldwide sales totaled approximately $2.8 billion, an increase of 5 percent on a reported basis, 3 percent on a constant currency basis and 2 percent on an operational basis as compared to the prior-year period. Operational sales adjust for the impact of foreign exchange, generic competition for U.S. cyclophosphamide, the Claris Injectables (Claris) acquisition and the previously communicated select strategic product exits the company is undertaking. In line with the financial outlook shared on October 25, 2017, fourth quarter revenues were negatively impacted by approximately $70 million as a result of temporary manufacturing disruptions in Puerto Rico due to the impact of Hurricane Maria.
Sales in the U.S. were $1.1 billion, increasing 1 percent. International sales totaled more than $1.6 billion, representing an 8 percent increase on a reported basis and a 4 percent increase on a constant currency basis.
Global sales for Hospital Products totaled $1.7 billion in the fourth quarter, advancing 5 percent on a reported basis, 3 percent on a constant currency basis and 1 percent operationally as compared to the prior-year period. Performance in the quarter benefited from continued strength in our U.S. fluid systems business as well as favorable demand for injectable pharmaceuticals, which includes the contribution of approximately $30 million of sales from the acquisition of Claris. Sales in the quarter also benefited from increased demand for the company’s advanced surgery products as well as cytotoxic contract manufacturing services. Hospital Products fourth quarter sales were negatively impacted by approximately $70 million as result of the Hurricane Maria related manufacturing disruptions.
Baxter’s fourth quarter Renal sales were approximately $1.1 billion, representing an increase of 5 percent on a reported basis, 3 percent on a constant currency basis and 4 percent operationally. Sales growth in Renal was driven by solid performance globally across both chronic and acute renal therapies.
Baxter reported a net loss from continuing operations of $61 million, or $0.11 per diluted share, on a GAAP (Generally Accepted Accounting Principles) basis for the fourth quarter. These results included special items totaling $137 million net ($415 million net after-tax, which includes a net tax charge of $322 million related to the estimated impact of U.S. tax reform). Other special charges in the quarter primarily included business optimization and intangible asset amortization.
On an adjusted basis, excluding special items, Baxter’s fourth quarter income from continuing operations totaled $354 million, or $0.64 per diluted share, exceeding the company’s previously issued guidance of $0.56 to $0.59 per diluted share.
Summary of Full-Year Results
Baxter’s worldwide sales totaled approximately $10.6 billion in 2017, an increase of 4 percent on both a reported and constant currency basis, and 5 percent on an operational basis as compared to the prior-year period. Sales within the United States totaled $4.5 billion, improving 6 percent on both a reported and operational basis over the prior year. International sales totaled approximately $6.1 billion, representing a 2 percent increase on a reported and constant currency basis and 4 percent operationally. Full-year sales for Hospital Products totaled $6.6 billion, reflecting growth of 5 percent on a reported, constant currency and operational basis. Baxter’s Renal sales totaled more than $3.9 billion, increasing 2 percent on both a reported and constant currency basis and 4 percent operationally.
For full-year 2017, Baxter reported income from continuing operations of $724 million, or $1.30 per diluted share, on a GAAP basis. These results include special items of $461 million net ($652 million net after-tax, including the estimated net impact of U.S. tax reform) primarily related to business optimization initiatives, intangible asset amortization, Claris integration expenses and deconsolidation of the company’s Venezuelan operations.
On an adjusted basis, excluding special items, Baxter’s full-year income from continuing operations totaled approximately $1.4 billion, or $2.48 per diluted share.
In 2017, Baxter generated $1.85 billion in operating cash flow, an increase of $229 million driven by improved operational performance and the continuing impact of programs focused on improving the company’s working capital. In addition, through disciplined management of expenditures, Baxter reduced capital spending by $85 million to $634 million. As a result, the company generated an increase of $314 million in free cash flow to $1.22 billion (operating cash flow less capital expenditures).
“Over the last two years, Baxter has demonstrated significant progress in improving our cash generation. This improvement allows the company flexibility to reinvest in the business and return value to shareholders through increased dividends and share repurchases,” said Jay Saccaro, Baxter’s chief financial officer. “During the year, we increased the annual dividend rate by 23 percent as well as repurchased $564 million worth of shares, while also pursuing internal and external initiatives to augment future growth.”
2017 Business Highlights
Baxter advanced its mission to save and sustain lives through patient-focused and customer-inspired innovation while generating profitable growth. Among the year’s highlights, the company:
- Completed its acquisition of Claris Injectables Limited, significantly broadening Baxter’s presence in the global generic injectable pharmaceuticals marketplace.
- Initiated high-potential research and development collaborations across a range of therapeutic opportunities with partners such as Mayo Clinic, Ramot at Tel Aviv University/Tel Aviv Sourasky Medical Center and ScinoPharm.
- Received U.S. Food and Drug Administration (FDA) guidance clarifying the regulatory pathway for a new home-based peritoneal dialysis (PD) solution generation system; and initiated U.S. clinical trials of HDx therapy enabled by THERANOVA, a Baxter technology currently available in other global markets that is designed to closely mimic the natural kidney through clearance of small to large middle molecules during dialysis.
- Introduced Baxter’s leading-edge PrisMAX acute care technology through a limited launch in select international markets; and also launched, in select markets in Europe, Middle East and Africa, a new indication for the oXIRIS set, the first 3-in-1 set for use in continuous renal replacement therapy (CRRT) and sepsis management protocols.
- Launched enhancements and upgrades for a range of Baxter products to promote safety and increase ease of use, including the AK 98 hemodialysis system, FLOSEAL Hemostatic Matrix, TISSEEL [Fibrin Sealant] and the SIGMA SPECTRUM infusion system.
As 2018 begins, Baxter remains focused on accelerating growth and innovation through both business development and its internal R&D pipeline. Among developments since the start of the year, the company:
- Announced entry into an agreement to acquire two surgical hemostat and sealant products from Mallinckrodt plc: RECOTHROM Thrombin topical (Recombinant), the first and only stand-alone recombinant thrombin, and PREVELEAK Surgical Sealant, which is used in vascular reconstruction, subject to the satisfaction of standard closing conditions.
- Received U.S. FDA approval of Bivalirudin in 0.9 percent Sodium Chloride Injection using Baxter’s proprietary frozen GALAXY container technology, making it the first and only presentation of Bivalirudin available in a convenient frozen premixed solution.
Puerto Rico Update
As noted in earlier press releases, regarding the impact of Hurricane Maria, Baxter experienced production disruptions at its three Puerto Rico facilities following challenges to the island’s power and infrastructure, which affected the manufacture of several products for the U.S. market. Baxter is committed to helping ensure patients have continued access to the products and therapies they need, and the company has been working diligently to restore its operations to full capacity following the storm.
All of Baxter’s Puerto Rico facilities are now back on the electrical grid and have been ramping up production to pre-hurricane levels. In addition, Baxter has been working closely with FDA, which granted regulatory discretion for temporary special importation of certain products from Baxter facilities in Ireland, Australia, Canada, Mexico, England, Italy and Brazil to help support product supply for the U.S. market. While the company expects to return to more normal supply levels for products manufactured in Puerto Rico over the coming weeks, sales in the first quarter will be negatively impacted by approximately $25 million.
“I am incredibly proud of and grateful to our employees around the world, who are inspired by our mission to save and sustain lives and have contributed in remarkable ways to our recovery efforts in Puerto Rico,” said Almeida. “I also want to thank our customers for their extraordinary patience and support as we work together through these unprecedented supply challenges, and to acknowledge the FDA’s valued assistance and partnership, which has been vital to the island’s continued recovery.”
2018 Financial Outlook
Baxter is providing its outlook for the full-year and first quarter of 2018:
- For full-year 2018, Baxter expects sales growth of 6 to 7 percent on a reported basis and approximately 4 percent on both a constant currency and operational basis. Operational sales adjust for the impact of foreign exchange, generic competition for cyclophosphamide and the benefit of the Claris acquisition. The company expects earnings from continuing operations, before special items, of $2.72 to $2.80 per diluted share.
- For the first quarter, the company expects sales growth of approximately 5 to 6 percent on a reported basis, or 1 to 2 percent on a constant currency basis. Operational sales are expected to be flat to up 1 percent as compared to the prior-year period. The company expects earnings from continuing operations, before special items, of $0.60 to $0.62 per diluted share.
The reconciliations between the projected 2018 adjusted diluted earnings per share and projected GAAP diluted earnings per share follows:
| 2018 Earnings per Share Guidance | Q1 2018 | FY 2018 |
|---|---|---|
| Diluted Earnings per Share - Adjusted | $0.60 - $0.62 | $2.72 - $2.80 |
| Estimated intangible asset amortization | $0.06 | $0.23 |
| Estimated business optimization | $0.07 - $0.09 | $0.19 - $0.24 |
| Diluted Earnings per Share - GAAP | $0.45 - $0.49 | $2.25 - $2.38 |
These estimates are based on information reasonably available at the time of this release, and future events or new information may result in different actual results.
A webcast of Baxter's fourth quarter 2017 conference call for investors can be accessed live from a link on the company's website at www.baxter.com beginning at 7:30 a.m. CST on February 1, 2018. Please see www.baxter.com for more information regarding this and future investor events and webcasts.
Segment Reporting Change
Beginning with the filing of the company’s Annual Report on Form 10-K, Baxter will report operating results based on three reportable geographic segments: Americas (North and South America), EMEA (Europe, Middle East and Africa) and APAC (Asia Pacific). Net sales will be reported based on these reportable segments, as well as the company’s new Global Business Units (GBUs). For comparative purposes, historical GBU information is currently available on our website.
About Baxter
Baxter provides a broad portfolio of essential renal and hospital products, including home, acute and in-center dialysis; sterile IV solutions; infusion systems and devices; parenteral nutrition; surgery products and anesthetics; and pharmacy automation, software and services. The company’s global footprint and the critical nature of its products and services play a key role in expanding access to healthcare in emerging and developed countries. Baxter’s employees worldwide are building upon the company’s rich heritage of medical breakthroughs to advance the next generation of healthcare innovations that enable patient care.
This release includes forward-looking statements concerning the company’s financial results, business development activities, capital structure, cost savings initiatives, R&D pipeline including results of clinical trials and planned product launches, and outlook for 2018 (including estimates regarding the timing of the company’s recovery from Hurricane Maria). The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: demand for and market acceptance of risks for new and existing products; product development risks; product quality or patient safety concerns; continuity, availability and pricing of acceptable raw materials and component supply; inability to create additional production capacity in a timely manner or the occurrence of other manufacturing or supply difficulties (including as a result of a natural disaster or otherwise); breaches or failures of the company’s information technology systems, including by cyber attack; future actions of regulatory bodies and other governmental authorities, including FDA, the Department of Justice, the New York Attorney General and foreign regulatory agencies; failures with respect to compliance programs; future actions of third parties, including payers; U.S. healthcare reform and other global austerity measures; pricing, reimbursement, taxation and rebate policies of government agencies and private payers; the impact of competitive products and pricing, including generic competition, drug reimportation and disruptive technologies; global, trade and tax policies; accurate identification of and execution on business development and R&D opportunities and realization of anticipated benefits (including the proposed acquisition of two surgical products from Mallinckrodt plc); the ability to achieve the intended results associated with the separation of the biopharmaceutical and medical products businesses; the ability to enforce owned or in-licensed patents or the patents of third parties preventing or restricting manufacture, sale or use of affected products or technology; the impact of global economic conditions; fluctuations in foreign exchange and interest rates; any change in law concerning the taxation of income (including current or future tax reform), including income earned outside the United States; actions taken by tax authorities in connection with ongoing tax audits; loss of key employees or inability to identify and recruit new employees; the outcome of pending or future litigation; the adequacy of the company’s cash flows from operations to meet its ongoing cash obligations and fund its investment program; and other risks identified in Baxter’s most recent filing on Form 10-K and other Securities and Exchange Commission filings, all of which are available on Baxter’s website. Baxter does not undertake to update its forward-looking statements.